Our laboratory focuses on the comprehensive study and development of separation processes, employing a multidisciplinary approach that combines molecular modeling, thermodynamic analysis, experimental techniques, and process simulation. Our research is driven by the need to design more efficient, sustainable, and innovative separation systems applicable across various sectors, including the chemical industry, food processing, and biotechnology. A core strength of our work lies in the use of molecular and thermodynamic tools to understand the behavior of complex systems at the microscopic level. We investigate phase equilibria, solute–solvent interactions, and activity coefficient models to accurately predict separation performance. These studies often involve the use of predictive models such as COSMO-RS and PC-SAFT, allowing us to screen solvents and systems in silico before experimental validation. This methodology has proven especially valuable in identifying suitable green solvents, such as ionic liquids and deep eutectic solvents, for the use in separation processes as shown in the following Figure.
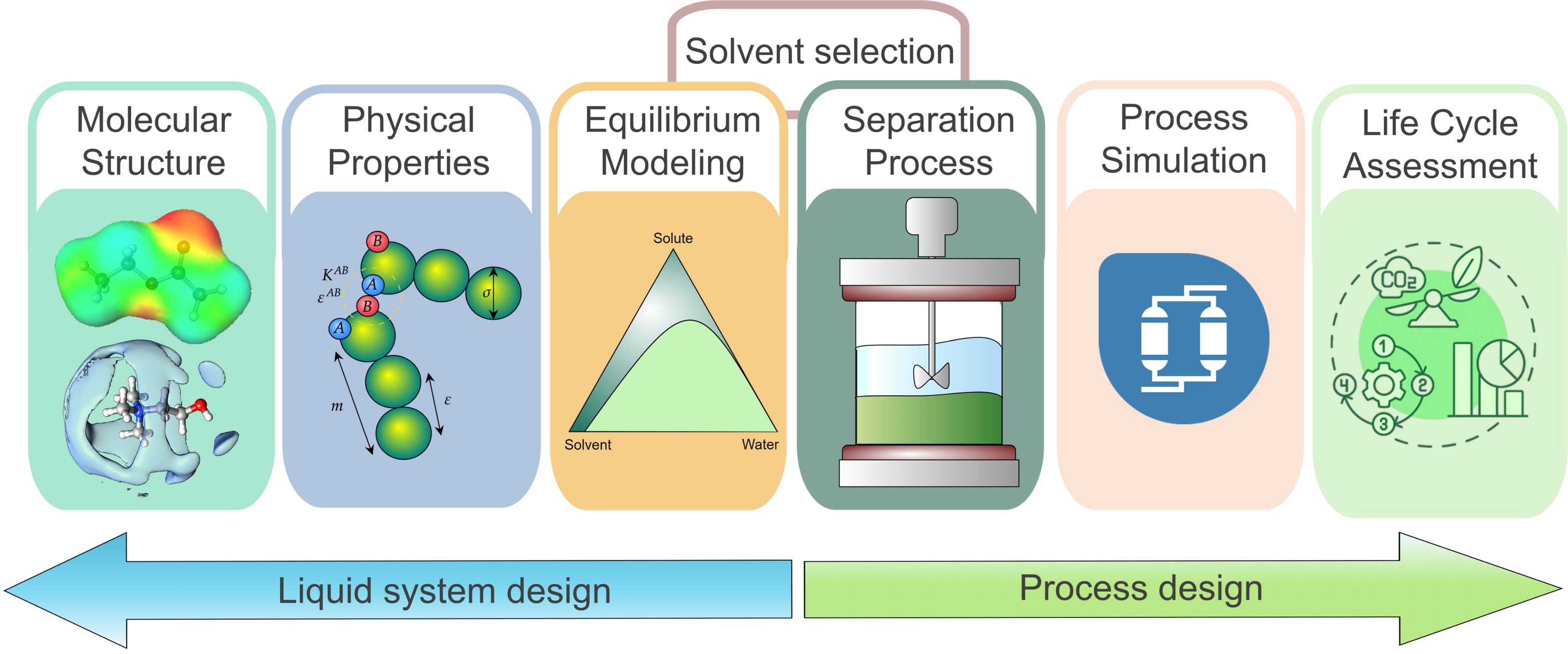
On the process side, we integrate experimental methods with process simulation platforms like Aspen Plus to evaluate and optimize separation units, such as distillation columns, membrane modules, and liquid-liquid extractors. These tools enable us to analyze energy demands, assess environmental impact, and propose design improvements that enhance the overall sustainability of industrial processes. Our experimental work provides critical data to validate simulation models and test new operational strategies, contributing to a more reliable understanding of real-world systems. Our research also extends into the food and biotechnology industries. In the food sector, we develop processes for the selective extraction and concentration of bioactive compounds, using environmentally friendly technologies such as aqueous two-phase systems and ultrasound-assisted extraction. These techniques are designed to retain the functional integrity of natural ingredients while reducing the reliance on organic solvents. In biotechnology, we focus on the extraction and stablization of proteins and enzymes, often exploring innovative biphasic systems and functionalized materials to improve yield and specificity.
What unifies our research across these diverse areas is the commitment to advance the science of separation through systematic integration of computational, experimental, and engineering approaches. Our recent efforts have explored hybrid methodologies that link thermodynamics with high-throughput screening and simulation, which is opening new frontiers in materials discovery and process intensification. Ultimately, our work contributes to the development of scalable, sustainable, and high-performance separation technologies that address critical challenges in modern industry. Whether enhancing the efficiency of chemical production, improving the nutritional quality of food products, or supporting the production of high-purity bioproducts, our laboratory remains at the forefront of innovation in separation process engineering.
Grant number: ACT240050 | Funding period: 2024-2027 | Investigators: Roberto Canales | Collaborators: Elodie Blanco, Néstor Escalona, Nicolás Gajardo-Parra
CO2 is one of the most abundant greenhouse gases, and it is targeted as the main contributor to the world’s climate change due to its increasing concentration in the atmosphere. Originally, CO2 had a natural cycle on Earth with a concentration below 300 ppm. However, its concentration passed the 400 ppm limit in 2018 due to anthropogenic activities, and the global temperature has increased by about 1.2 °C since 1850. This situation has brought a series of events to the earth’s surface. For instance, extreme temperatures, flooded areas, changes in crop seasons, and pH modification on the sea due to CO2 absorption, among many others. To deaccelerate the CO2 concentration growth in the atmosphere, the Paris Agreement proposed a goal of a temperature increase no higher than 2 °C by 2050, with a more ambitious objective of 1.5°C. Then, carbon neutrality needs to be reached by 2050. Chile has taken several measures to reach carbon neutrality by 2050. For example, the Government proposed reducing or eliminating coal or fuel-fired thermoelectrical plants or applying CO2 capture technologies if they are still in use or new related projects are applied. Under this idea, carbon neutrality is projected to 2050 because of the CO2-absorbing forestal plantations and native forests in the south of Chile. However, industrial sectors are not expected to reduce their emissions drastically in the near future, so there is an opportunity for applying CO2 capture techniques in this sector for its further utilization towards the formation of interesting products that are not widely produced in the country, like methanol, and hydrocarbons. This is possible because the policies incentivizing the future availability of green H2 in the country could allow the synthesis of the aforementioned products by hydrogenating CO2. The main challenge in obtaining these products is that capture technologies are not applied in Chile for CO2 availability, and there are not many plants implemented at an industrial scale worldwide for this purpose. Moreover, hydrogenation techniques require catalysts that still need improvements for performing a process with economic feasibility.
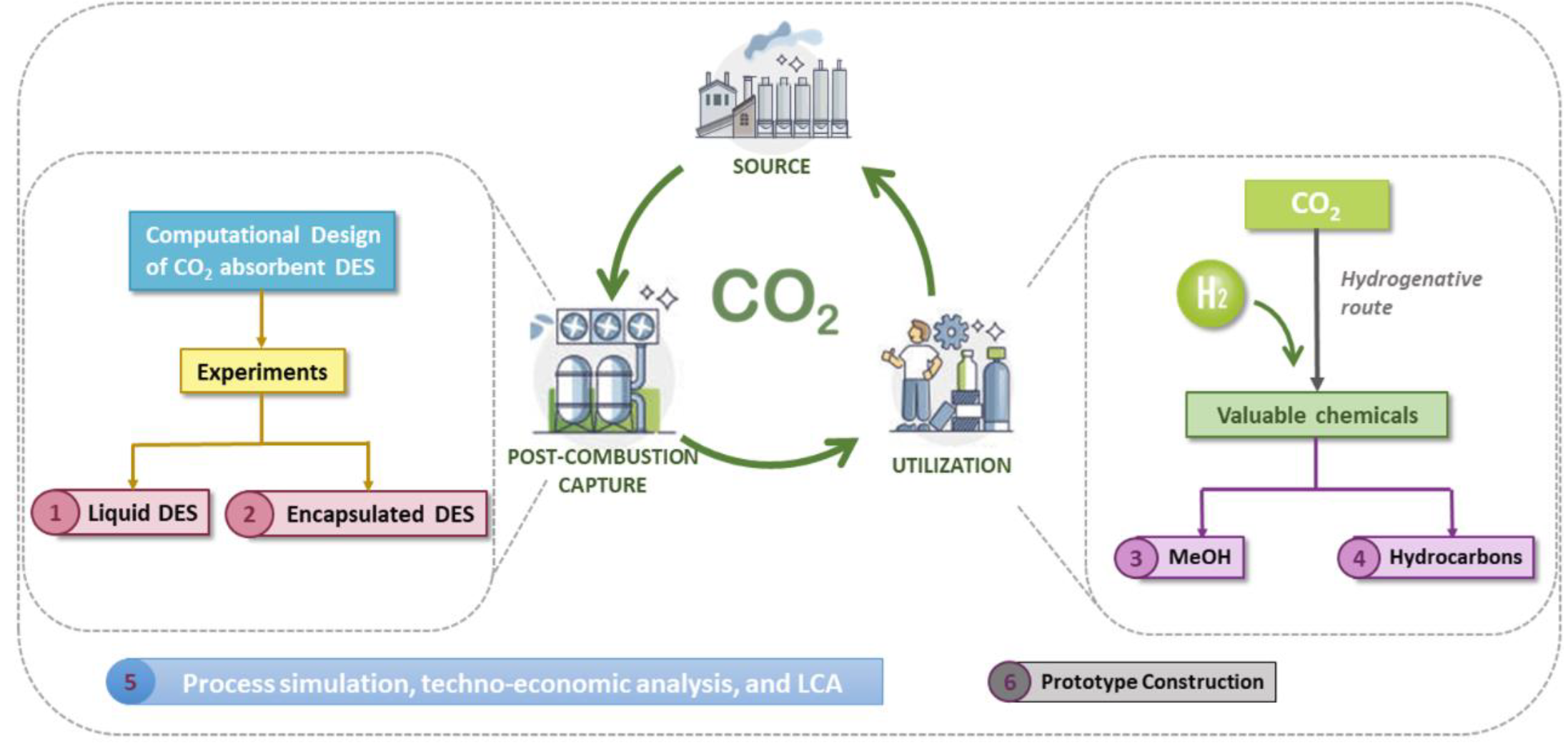
This work aims to study potential liquid absorbents that could effectively capture CO2 from post-combustion industrial sources. These sorbents should be able to release CO2 for its further use in the hydrogenation reactions without losing their capability to be reused in a new capture/release cycle. Absorbents that show these potential capabilities are functionalized deep eutectic solvents in liquid form or encapsulated. In the second stage, novel catalysts based on NiGa/CeOx will be studied to hydrogenate CO2 into methanol, and functionalized graphene oxides will be targeted as attractive catalysts for producing hydrocarbons. A process design of the capture and conversion alternatives, along with a techno-economic analysis, will be performed for studying feasible scaling options. Also, a life-cycle assessment is expected for analyzing the deep eutectic solvents and catalysts used for capture and conversion, respectively, and also for the processes investigated. Finally, using the previous information, a prototype of the optimized CO2 capture unit coupled to the CO2 conversion unit will be set up at laboratory scale. Then, investigation in TRL 3 will finish at TRL 4 after accomplishing the objectives of this project.
Grant number: 1240931 | Funding period: 2024-2028 | Investigators: Roberto Canales | Collaborators: René Cabezas
The market of flavors and fragrances is always increasing because of the diversification of their applications in food, cosmetics, perfumes, pharmaceuticals, household products, etc. Chemical synthetic aromas cover an important part of this market because of the low production costs. Despite the purity and natural-like grade of synthetic products, the perception of consumers is towards purchasing products that are natural and potentially free of any undesirable compound. This leads to a very high increase in the price of natural products compared with their synthetic alternative, even when the second is labeled as natural-like. An interesting alternative to chemically produced flavors and fragrances is biotechnological synthesis, specifically, the fermentation with specially selected yeasts that overproduce the target solute. This process is considered natural, but there are several challenges to overcome. For example, in most cases, productivity is still low, some undesirable side products can be obtained, and one of the main issues is that the product could be toxic to the yeast, inhibiting its formation in higher concentrations. To overcome the toxicity problem, an in-situ product removal (ISPR) technique is recommended for separating the product as soon as it is produced. A promising ISPR technique is solute removal with a hydrophobic and biocompatible solvent by liquid-liquid extraction. However, extraction with solvents is the most studied technique and presents several advantages like simplicity, good distribution ratios, selectivity, etc. The most difficult part is the selection of the optimal solvent because it must be scarcely soluble in water, biocompatible with the yeast, and recover high amounts of product. Thus, a membrane-assisted liquid-liquid extraction could avoid mainly the biocompatibility issue. Another challenge is the purification of the aroma by separating it from the solvent after the extraction and the recovery of the solvent for another extraction cycle.
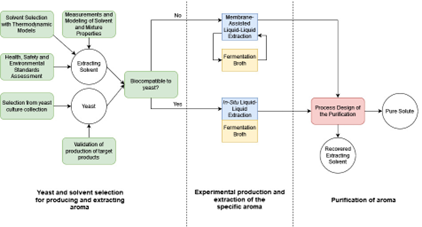
There are two flavors and fragrances of industrial interest that can be produced by fermentation and selected for this proposal: 2-phenylethanol, and -decalactone. The work methodology is divided into three main steps: (1) selection of yeast and the extracting solvent, (2) solute extraction from the fermentation broth via in-situ or membrane-assisted liquid-liquid extraction using the solvent selected in step (1), and (3) solute purification and solvent recovery. For step (1), the objective is to select yeasts provided by the Phaff Yeast Culture Collection from The University of California Davis to improve the production of the aroma and combine a predictive selection of solvents using COSMO-RS with Health, Safety, and Environmental guides for assessing potential extractive solvents. Predictive selection will be validated with experimental liquid-liquid equilibrium measurements and vapor-liquid equilibrium measurements are performed for feeding step (3). Once the solvents are selected in the previous step, they need to pass a biocompatibility test with the yeasts used for producing the aromas, to assess their applicability in the real in-situ extraction from the fermentative system, otherwise, they can be used in a membrane-assisted liquid-liquid extraction. Thus, for step (2) the selected solvents will be tested for extracting the aromas in the real fermentative system. Finally, for step (3) the study of the aroma + solvent mixture will be performed to understand if they can be separated by distillation to purify the aroma and recover the solvent for a new extraction cycle. This study will be performed from a process design by simulation in Aspen Plus using COSMO-based models or PC-SAFT as the thermodynamic tool for liquid-liquid extraction and the distillation steps. Finally, the best solvents are prioritized with an analytical hierarchy process based on sustainability indicators from the simulation.
| Grant number: 3250050 | Funding period: 2025-2028 | Investigators: Nicolás Gajardo-Parra | Collaborators: Nadia Guajardo |
The fundamentals behind the effects of liquid phase properties on reaction performance are often not rationalized in detail. Instead, decision steps in (bio)chemical process design are generally based on empirical knowledge, lacking a systematic decision framework. The main objective of this project is to select a optimal solvent for the development of a sustainable biocatalytic systems to obtain biolubricants through an enzymatic esterification reaction. The project will use a commercially available immobilized lipase for the biocatalytic esterification of 5-(hydroxymethyl)furfural.
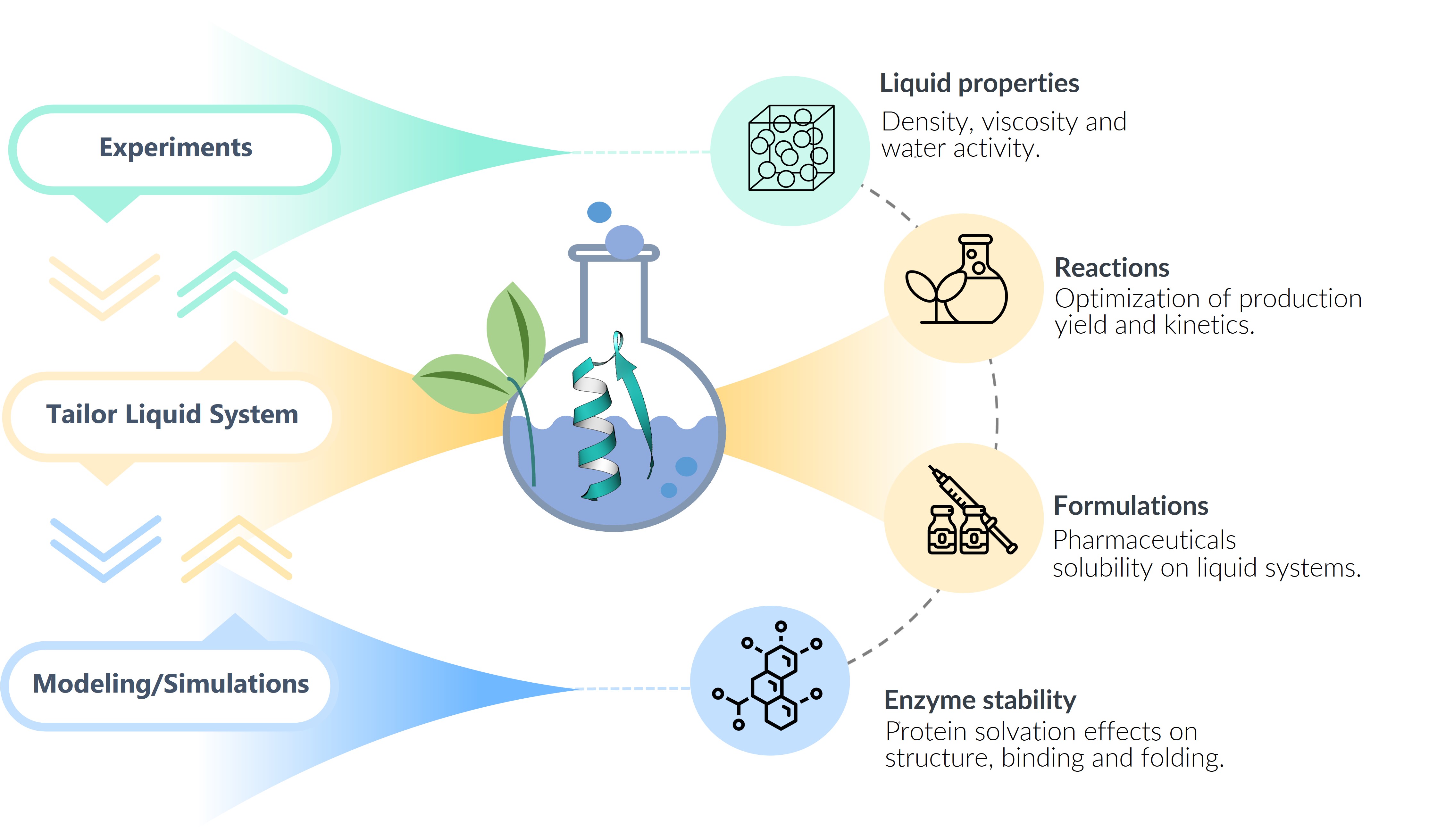
The project seeks to propose a (1) tailored liquid systems that will boost or even enable complex (bio)chemical reactions and downstream processing optimization and a (2) tailored liquid system that guarantee the stability and targeted functionality of biological catalyst. The project’s first aim is to advance knowledge-enabled design of well-defined liquid model systems, which requires that the underlying phenomena are understood on a molecular level. For this, thermodynamics models such as COSMO-RS and PC-SAFT will be used to understand the interactions within the liquid system and docking or molecular dynamics simulations to understand the interactions between the protein and the liquid system. The development of physics-based models for liquid systems is hindered by the scarcity of experimental data reporting on molecular interactions and molecular structure in these systems. Once promising liquid systems are selected, their effect on kinetic and equilibrium properties of the biocatalytic reaction will be experimentally tested, focusing mainly on concentration and water effects. This will allow the models to be adjusted to the behavior of the reaction to optimize the downstream process using a two-phase system. This will allow the creation of a toolbox that allows predicting the effect of various solvents on the esterification reaction, reducing the need for costly trial-and-error experiments for the selection of solvents in this reaction and optimizing a reaction with high industrial potential in Chile adding value to the lignocellulose residues.